Home » Course Layouts » Free Course Layout Udemy
Engineering Thermodynamics. Instructor: Dr. Jayant K. Singh, Department of Chemical Engineering, IIT Kanpur.
0
English
English [CC]
- Learn basic syntax that can apply to any language.
- Learn what is a programming language and the basic concepts for beginners.
- Understand what is Javascript in it's truest form.
- Know the basic syntax of Javascript.
- Know some hidden quirks in Javascript.
Description
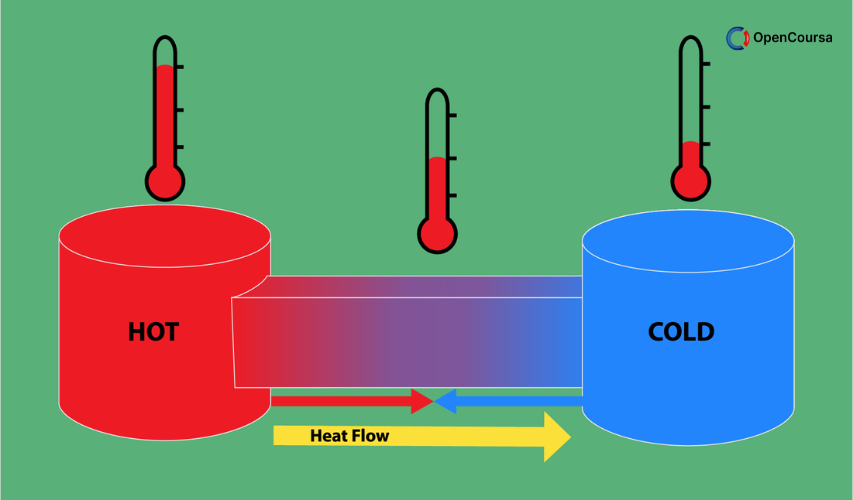
This course provides an introduction to the most powerful engineering principles -Thermodynamics: the science of energy and its transformation from one form to another form. The subject is widely applicable in several branches of engineering and science. The objective of this course is to introduce systematic different tools needed to analyze energy systems from various daily lives to large scale engineering applications. More specifically, we will cover the topics of mass and energy conservation principles; first law analysis of closed and open systems; understanding second law of thermodynamics and entropy; exergy; properties of pure substances; power generation and refrigeration on thermodynamic cycles; thermodynamic relation, combustion and reaction. (from nptel.ac.in)
Course content
-
- Lecture 01 – Fundamental Laws of Nature, System Definitions and Applications Unlimited
- Lecture 02 – Thermodynamic Property, State, Equilibrium, Process Unlimited
- Lecture 03 – Temperature Scale and Pressure Unlimited
- Lecture 04 – Macroscopic and Microscopic Forms of Energy Unlimited
- Lecture 05 – Different Forms of Work, Energy Transfer and Sign Convention Unlimited
- Lecture 06 – First Law of Thermodynamics and Energy Balance Unlimited
- Lecture 07 – Efficiency of Mechanical and Electrical Devices Unlimited
- Lecture 08 – Examples on Basic Concepts and Energy Balance Unlimited
-
- Lecture 09 – Phase Change of a Pure Substance Unlimited
- Lecture 10 – Property Diagrams of Pure Substances Unlimited
- Lecture 11 – Thermodynamic Properties of a Pure Substance from a Property Table Unlimited
- Lecture 12 – Thermodynamic Properties of a Pure Substance Unlimited
- Lecture 13 – Equations of State and Compressibility Chart Unlimited
- Lecture 14 – Example Problems on Properties of Pure Substances Unlimited
- Lecture 15 – Quasi-equilibrium, Moving Boundary Work Unlimited
- Lecture 16 – Polytropic Process Unlimited
- Lecture 17 – Energy Analysis of Closed System, Unrestrained Expansion Unlimited
- Lecture 18 – Internal Energy, Enthalpy, and Specific Heats of Ideal Gas Unlimited
- Lecture 19 – Internal Energy, Enthalpy, and Specific Heats of Solids and Liquids Unlimited
- Lecture 20 – Examples on Energy Balance for Closed Systems and Moving Boundary Work Unlimited
- Lecture 27 – Second Law of Thermodynamics, Heat Engine and Cyclic Devices Unlimited
- Lecture 28 – COP of Refrigerator and Heat Pump, Second Law Statements Unlimited
- Lecture 29 – Perpetual Motion Machines, Reversible and Irreversible Processes, Carnot Cycle Unlimited
- Lecture 30 – Carnot Principles, Thermodynamic Temperature Scale, Carnot HE and HP Unlimited
- Lecture 31 – Examples on the Second Law of Thermodynamics Unlimited
- Lecture 32 – Clausius Inequality, Applications of the Second Law of Thermodynamics Unlimited
- Lecture 33 – Entropy, Increase in Entropy Principle, Isentropic Process Unlimited
- Lecture 34 – Change in Entropy of Solids, Liquids, and Ideal Gases Unlimited
- Lecture 35 – Reversible Flow Work, Multistage Compressor, Efficiency of Pump and Compressors Unlimited
- Lecture 36 – Entropy Balance in Closed System and Control Volume Unlimited
- Lecture 37 – Examples related to Entropy Change in a System Unlimited
- Lecture 43 – Gas Power Cycle, Air Standard Assumptions, Value of Carnot Cycle in Engineering Unlimited
- Lecture 44 – An Overview of Reciprocating Engine Otto Cycle Unlimited
- Lecture 45 – Analysis of Diesel Cycle Unlimited
- Lecture 46 – Analysis of Brayton Cycle Unlimited
- Lecture 47 – Examples on Gas Power Cycles such as Otto Diesel and Brayton Unlimited
- Lecture 48 – Rankine and Carnot Vapor Power Cycles Unlimited
- Lecture 49 – Ideal Regenerative Rankine Cycle, Cogeneration, Combined Gas Vapor Cycle Unlimited
- Lecture 50 – Refrigeration Cycles Unlimited
- Lecture 51 – Examples on Vapour Power Cycles Unlimited
N.A
- 5 stars0
- 4 stars0
- 3 stars0
- 2 stars0
- 1 stars0
No Reviews found for this course.