Home » Course Layouts » Free Course Layout Udemy
This course provides an introduction to the chemistry of biological, inorganic, and organic molecules.
0
1
English
English [CC]
- Learn basic syntax that can apply to any language.
- Learn what is a programming language and the basic concepts for beginners.
- Understand what is Javascript in it's truest form.
- Know the basic syntax of Javascript.
- Know some hidden quirks in Javascript.
Description
The emphasis is on basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis.
In an effort to illuminate connections between chemistry and biology, a list of the biology-, medicine-, and MIT research-related examples used in 5.111 is provided in Biology-Related Examples.
Acknowledgements
Development and implementation of the biology-related materials in this course were funded through an HHMI Professors grant to Prof. Catherine L. Drennan. Videos and captioning were made possible and supported by the MIT Class of 2009.
Course content
- Lecture 1: The Importance of Chemical Principles Unlimited
- Lecture 2: Discovery of Electron and Nucleus Unlimited
- Lecture 3: Wave-Particle Duality of Light Unlimited
- Lecture 4: Wave-Particle Duality of Matter Unlimited
- Lecture 5: Hydrogen Atom Energy Levels Unlimited
- Lecture 6: Hydrogen Atom Wavefunctions Unlimited
- Lecture 7: P-Orbitals Unlimited
- Lecture 8: Multielectron Atoms and Electron Configurations Unlimited
- Lecture 9: Periodic Trends Unlimited
- Lecture 10: Covalent Bonds Unlimited
- Lecture 11: Lewis Structures Unlimited
- Lecture 12: Ionic Bonds Unlimited
- Lecture 13: Polar Covalent Bonds and VSEPR Theory Unlimited
- Lecture 14: Molecular Orbital Theory Unlimited
- Lecture 15: Valence Bond Theory and Hybridization Unlimited
- Lecture 16: Thermochemistry Unlimited
- Lecture 17: Entropy and Disorder Unlimited
- Lecture 18: Free Energy and Control of Spontaneity Unlimited
- Lecture 19: Chemical Equilibrium Unlimited
- Lecture 20: Le Chatelier’s Principle Unlimited
- Lecture 21: Acid-Base Equilibrium Unlimited
- Lecture 22: Chemical and Biological Buffers Unlimited
- Lecture 23: Acid-Base Titrations Unlimited
- Lecture 24: Balancing Redox Equations Unlimited
- Lecture 25: Electrochemical Cells Unlimited
- Lecture 26: Chemical and Biological Redox Reactions Unlimited
- Lecture 27: Transition Metals Unlimited
- Lecture 28: Crystal Field Theory Unlimited
- Lecture 29: Metals in Biology Unlimited
- Lecture 30: Magnetism and Spectrochemical Theory Unlimited
- Lecture 31: Rate Laws Unlimited
- Lecture 32: Nuclear Chemistry and Elementary Reactions Unlimited
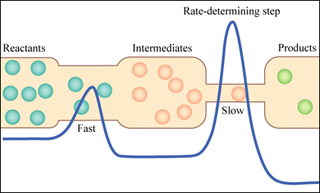
- Lecture 33: Reaction Mechanism Unlimited
- Lecture 34: Temperature and Kinetics Unlimited
- Lecture 35: Enzyme Catalysis Unlimited
- Lecture 36: Biochemistry Unlimited
N.A
- 5 stars0
- 4 stars0
- 3 stars0
- 2 stars0
- 1 stars0
No Reviews found for this course.